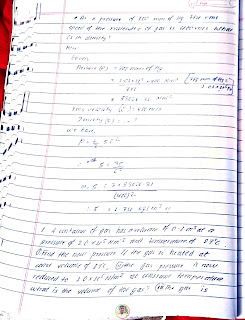Syllabus:
- Explain the molecular concept of thermal energy, heat and temperature, and cause and direction of heat flow
- Explain the meaning of thermal equilibrium and Zeroth law of thermodynamics.
- Explain thermal equilibrium as a working principle of mercury thermometer.
- Explain some examples and applications of thermal expansion, and demonstrate it with simple experiments.
- Explain linear, superficial, cubical expansion and define their corresponding coefficients with physical meaning.
- Establish a relation between coefficients of thermal expansion.
- Describe Pullinger’s method to determine coefficient of linear expansion
- Explain force set up due to expansion and contraction.
- Explain differential expansion and its applications.
- Explain the variation of density with temperature.
- Explain real and apparent expansion of liquid appreciating the relation (gamma)r = (gamma)g + (gamma)a.
- Describe Dulong and Petit’s experiment to determine absolute expansivity of liquid.
- Solve mathematical problems related to thermal expansion.
- Define heat capacity and specific heat capacity and explain application of high specific heat capacity of water and low specific heat capacity of cooking oil and massage oil
- Describe Newton’s law of cooling with some suitable daily life examples.
- Explain the principle of calorimetry and describe any one standard process of determining specific heat capacity of a solid
- Explain the meaning of latent heat of substance appreciating the graph between heat and temperature and define specific latent heat of fusion and vaporization.
- Describe any one standard method of measurement of specific latent heat of fusion and explain briefly the effect of external pressure on boiling and melting point.
- Distinguish evaporation and boiling.
- Define triple point.
- Solve mathematical problems related to heat
- Explain the transfer of heat by conduction, convection and radiation with examples and state their applications in daily life.
- Define temperature gradient and relate it with rate of heat transfer along a conductor.
- Define coefficient of thermal conductivity and describe Searl’s method for its determination.
- Relate coefficient of reflection (r), coefficient of transmission (t) and coefficient of absorption (r + a + t = 1).
- Explain ideal radiator (e= 1, a =1) and black body radiation.
- State and explain Stefan’s law of black body radiation using terms; emissive power and emissivity.
- Describe idea to estimate apparent temperature of sun.
- Solve mathematical problems related to thermal conduction and black body radiations.
- Relate pressure coefficient and volume coefficient of gas using Charles’s law and Boyle’s law.
- Define absolute zero temperature with the support of P - V, V- T graph.
- Combine Charles’s law and Boyle’s law to obtain ideal gas equation.
- Explain molecules, inter molecular forces, moles and Avogadro’s number.
- Explain the assumptions of kinetic – molecular model of an ideal gas.
- Derive expression for pressure exerted by gas due to collisions with wall of the container appreciating the use of Newton’s law of motion.
- Explain the root mean square speed of gas and its relationship with temperature and molecular mass.
- Relate the pressure and kinetic energy.
- Calculate the average translational kinetic energy of gas for 1 molecule and Avogadro’s number of molecules.
- Solve mathematical problems related ideal gas.